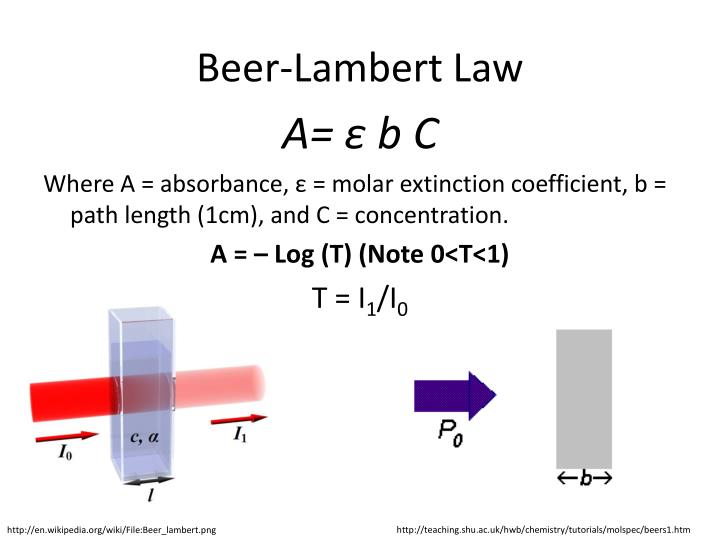
Beer Lambert Law For Solids . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. German scientist august beer described a separate attenuation relation in 1852. If this light can be. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. You can also use this.
from www.slideserve.com
German scientist august beer described a separate attenuation relation in 1852. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). If this light can be. You can also use this. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
PPT Determining the Concentration of a Solution Beer’s Law
Beer Lambert Law For Solids German scientist august beer described a separate attenuation relation in 1852. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. You can also use this. If this light can be. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). lambert’s law says the absorbance of a sample is directly proportional to the path length of light. German scientist august beer described a separate attenuation relation in 1852. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law For Solids lambert’s law says the absorbance of a sample is directly proportional to the path length of light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). German scientist august beer described a separate attenuation relation in 1852. If this light can be. the amount of light that a species absorbs in. Beer Lambert Law For Solids.
From www.youtube.com
BeerLambert law in easy way YouTube Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. You can also use this. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). German scientist august beer described a separate attenuation relation in 1852. lambert’s law says the. Beer Lambert Law For Solids.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. German scientist august beer described a separate attenuation relation in 1852. You can also use this. It provides a. Beer Lambert Law For Solids.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law For Solids It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. If this light can be. You can also use this. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. lambert’s law says the absorbance of a sample is directly proportional. Beer Lambert Law For Solids.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law For Solids German scientist august beer described a separate attenuation relation in 1852. You can also use this. If this light can be. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\).. Beer Lambert Law For Solids.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law For Solids If this light can be. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. German scientist august beer described a separate attenuation relation in 1852. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). the amount of light that a species absorbs in. Beer Lambert Law For Solids.
From studylib.net
Beer Lambert Law Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. German scientist august beer described a separate attenuation relation in 1852. If this light can be. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Beer stated the transmittance of. Beer Lambert Law For Solids.
From www.researchgate.net
3. Illustration to Beerlambert's law. Download Scientific Diagram Beer Lambert Law For Solids It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). lambert’s law says the absorbance of a sample is directly proportional to the path length of light. Beer stated the transmittance of a solution is. Beer Lambert Law For Solids.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer Lambert Law For Solids Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). lambert’s law says the absorbance. Beer Lambert Law For Solids.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law For Solids lambert’s law says the absorbance of a sample is directly proportional to the path length of light. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Beer Lambert Law For Solids.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer Lambert Law For Solids You can also use this. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. If this light can be. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer stated the transmittance of a solution is constant if the product of the. Beer Lambert Law For Solids.
From www.youtube.com
B.7 The Beer Lambert law (HL) YouTube Beer Lambert Law For Solids It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. If this light can be. You can also use this. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. German scientist august beer described a separate attenuation relation in 1852. the amount. Beer Lambert Law For Solids.
From www.researchgate.net
1 BeerLambert law; Xrays to solid matter interaction. Download Beer Lambert Law For Solids lambert’s law says the absorbance of a sample is directly proportional to the path length of light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light. Beer Lambert Law For Solids.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law For Solids If this light can be. German scientist august beer described a separate attenuation relation in 1852. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). You can also use this. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.. Beer Lambert Law For Solids.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). If this light can be. German scientist august beer described a separate attenuation relation in 1852. You can also use this.. Beer Lambert Law For Solids.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. You can also use this. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. If this light can be. German scientist august beer described a separate. Beer Lambert Law For Solids.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law For Solids It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. You can also use this. lambert’s law says the absorbance of a sample is directly proportional to the path length of light. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are. Beer Lambert Law For Solids.
From www.slideserve.com
PPT Lab 6 Saliva Practical BeerLambert Law PowerPoint Presentation Beer Lambert Law For Solids the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light can be. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). It provides a mathematical relationship between the substance’s concentration in a solution and its ability to. Beer Lambert Law For Solids.